Abstract
Background: Fludarabine (Flu) is one of the most common lymphodepleting chemotherapy (LDC) agents given before CD19 CAR T cells. Because the main pharmacokinetic (PK) predictors of Flu exposure are weight and glomerular filtration rate (GFR) and not body-surface area (BSA), standard BSA-dosed Flu leads to highly variable drug exposure among patients (pts) (Langenhorst et al., 2019). We hypothesized that variable Flu exposure influences outcomes for pts with relapsed/refractory (rel/ref) aggressive B-NHL receiving CD19 CAR T cells (Fabrizio et al.; Dekker et al., Blood Advances 2022). To test this, we estimated Flu exposure and evaluated its association with key outcomes, aiming to identify a target exposure that optimized efficacy and tolerability.
Methods: This is an IRB-approved retrospective study using data from the Cell Therapy Consortium (CTC), a multicenter working group/registry of 8 U.S. academic institutions performing real-world, observational studies on pts receiving commercial CAR T cells. This analysis included adults (≥18 y/o) with rel/ref aggressive B-NHL treated with axicabtagene ciloleucel (axi-cel) between 4/2018-6/2021. Eligible pts received LDC per the FDA label: Flu 30 mg/m2 & cyclophosphamide 500 mg/m2 daily x 3 days starting day -5. Exclusions included: weight unsupported by the PK model (>125 kg), >3-days between end of LDC and cell infusion, and/or LDC given on an interrupted schedule. We estimated cumulative Flu exposure (AUC; mg*h/L) for each pt using a validated population PK model and the following variables: GFR, actual body weight, height, and the daily doses (mg) of Flu given (Langenhorst et al., 2019). Main outcomes were PFS, OS, and cumulative incidences (CIs) of rel/POD, CRS, and ICANS. To define an optimal Flu exposure, univariable (UV) hazard ratio (HR) plots were drawn with P-splines, and exposure thresholds were set by assessing where the HRs (and confidence intervals) crossed to <1. The chosen Flu exposure thresholds were used as categorical variables in UV and multivariable (MV) Cox proportional models.
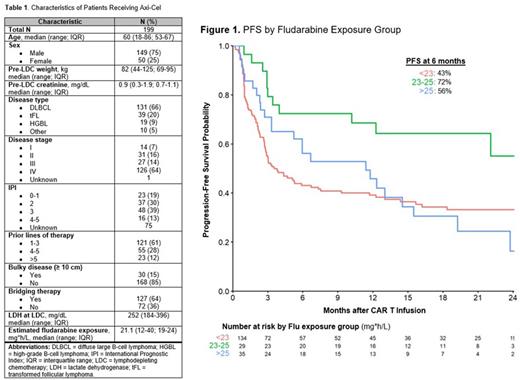
Results: Characteristics of the 199 included pts are shown in Table 1. Among all pts, median estimated Flu exposure was 21.1 (range 12-40, IQR 19-24). Three Flu exposure thresholds of <23 (N=134), 23-25 (N=29), and >25 (N=35) were selected for analyses. Median follow-up was 18.4 months (mos). CIs of rel/POD at 6 mos by exposure group were 53% (44-61%), 27% (12-45%), and 32% (17-48%), respectively. PFS rates at 6 mos were 43% (35-52%), 72% (58-91%), and 56% (42-76%), respectively (Figure 1). OS rates at 6 mos were 70% (62-78%), 86% (75-100%), and 68% (54-85%), respectively. In each exposure group, 64, 8, and 18 pts died, respectively. In the <23 group, causes of death (COD) were rel/POD (N=57; 89%), infection (N=3; 5%), and toxicity (N=4; 6%); in the 23-25 group COD were rel/POD (N=7; 87%) and infection (N=1; 12%); and in the >25 group COD were rel/POD (N=8; 44%), infection (N=4; 22%), and toxicity (N=5; 28%) with 3 of these deaths due to ICANS.
In UV analyses (not shown), Flu exposure of 23-25 was significantly associated with the lowest risk of rel/POD, and highest PFS and OS compared to the other groups. Flu exposure of <23 was associated with the highest risk of rel/POD and lowest PFS, while an exposure of >25 was associated with comparable PFS and OS to <23. Flu exposure was not associated with risk of CRS, but exposure >25 was associated with a higher risk of any-grade ICANS. In MV analyses, Flu exposure of 23-25 was associated with the highest PFS (HR 0.48; 0.26-0.91, p=0.02) and lowest risk of rel/POD (HR 0.5; 0.28-0.96, p=0.04) without an increased risk of any-grade ICANS (HR 1.5; 0.86-2.5, p=0.16). Higher LDH and bulky disease were associated with poorer PFS and higher rates of rel/POD. Flu exposure of >25 was associated with the highest risk of any-grade ICANS (HR 1.6; 1.1-2.5, p=0.03).
Conclusions: These CTC registry findings suggest that there is a Flu exposure target window associated with improved survival in pts with rel/ref aggressive B-NHL receiving axi-cel. Flu underexposure is associated with a higher risk of disease-related treatment failure while overexposure is associated with a higher risk of toxicities. This optimal Flu exposure is achievable through personalized, PK-directed dosing and is a novel, easily modifiable strategy to improve outcomes after CAR T cell therapy. These data require further external validation which is currently on-going.
Disclosures
Scordo:Kite - A Gilead Company: Other: Ad-hoc advisory board (past); Amgen, Inc.: Research Funding; Omeros Corporation: Consultancy, Research Funding; Medscape, LCC (CME): Honoraria; i3Health (CME): Honoraria; McKinsey & Company: Consultancy; Angiocrine Bioscience, Inc.: Consultancy, Research Funding. Shouval:Medexus: Consultancy, Ended employment in the past 24 months; MyBiotics: Consultancy. Porter:Janssen: Consultancy; Jazz: Consultancy; Incyte: Consultancy; Gerson Lerhman Group: Consultancy; Adecept Bio: Consultancy; DeCART: Consultancy; BMS: Consultancy; Bluebird Bio: Consultancy; Kadmon: Consultancy; Angiocrine: Consultancy; Mirror Biologics: Consultancy; Genentech: Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company; Tmunity Therapeutics: Patents & Royalties: anti-CD19 CART; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Wiley: Honoraria; Elsevier: Honoraria; Kite/Gilead: Consultancy; Novartis: Consultancy, Patents & Royalties: anti-CD19 CART, Research Funding. Schuster:DTRM: Research Funding; Adaptive Biotechnologies: Research Funding; Abbvie: Research Funding; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Nanovector: Consultancy; Nordic: Consultancy; MustangBio: Consultancy; Morphosys: Consultancy; Loxo: Consultancy; Legend Biotech: Consultancy; Janssen: Consultancy; Incyte: Consultancy, Research Funding; Genetech/Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; BiGene: Consultancy; Acerta: Consultancy; Juno Therapeutics: Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; TG Therapeutics: Research Funding. Bachanova:Incyte: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Citius Pharma: Research Funding; FATA Therapeutics: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Karyopharma: Consultancy; Gamida Cell: Membership on an entity's Board of Directors or advisory committees, Research Funding. Maakaron:Gilead: Research Funding; CRISPR Therapeutics: Research Funding; Precision BioSciences: Research Funding; Scripps: Research Funding; Fate Therapeutics: Research Funding; ADC Therapeutics: Research Funding. Maziarz:Orca Bio: Other: Support for research analysis and for medical writing; Novartis: Other: Support for research on CART; Allovir: Other: Support for research on Allo HCT costs of care of infectious related complications; ASTCT: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Consultancy, Honoraria. Nastoupil:ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; Genentech/Roche, MEI, Takeda: Other: DSMC; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding. McGuirk:Orca Bio: Research Funding; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Speakers Bureau; Nextar: Consultancy, Honoraria; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial. Oluwole:ADC Therapeutics: Consultancy; Kite, a Gilead Company: Research Funding; Janssen: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Curio Science: Consultancy; TG Therapeutics: Consultancy. Ip:Seagen: Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. Leslie:Karyopharm: Speakers Bureau; Eli Lily: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Jansssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Celegene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Speakers Bureau; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support. Bishop:Immatics: Research Funding; Arcellx: Consultancy, Research Funding; Triumvira: Research Funding; WindMIL Therapeutics: Consultancy; Autolus: Consultancy, Research Funding; Tmunity: Research Funding; Incyte: Honoraria, Other: Travel support , Speakers Bureau; Bluebird Bio: Consultancy; Iovance: Consultancy; CRISPR Therapeutics: Consultancy, Research Funding; Agios: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; Bristol Myers Squibb: Honoraria, Other: Travel support, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Travel support , Research Funding; Sanofi: Honoraria, Speakers Bureau; Celgene: Honoraria; Chimeric Therapeutics: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau; Sana Biotechnology: Consultancy; ADC Therapeutics: Speakers Bureau; Servier: Speakers Bureau. Riedell:MorphoSys: Research Funding; Tessa Therapeutics: Research Funding; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Research Funding; Xencor: Research Funding; Calibr: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sana Biotechnology: Consultancy. Perales:Merck: Consultancy; Nektar Therapeutics: Consultancy, Honoraria; Cidara Therapeutics: Consultancy; Sellas Life Sciences: Consultancy; Abbvie: Honoraria; Astellas: Honoraria; Celgene: Honoraria; Karyopharm: Honoraria; MorphoSys: Consultancy, Honoraria; Vor Biopharma: Honoraria; VectivBio AG: Honoraria; Omeros: Consultancy; Orca Bio: Consultancy; Miltenyi Biotec: Consultancy, Honoraria; Novartis: Honoraria; Kite, a Gilead Company: Honoraria, Research Funding; Medigene: Consultancy; Servier: Consultancy; Bellicum: Honoraria; DSMB: Other; Incyte: Honoraria, Research Funding; Bristol-Mysers Squibb: Honoraria; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal